D-Glucose: Glucose, a simple sugar with the chemical formula C6H12O6, is a vital molecule for all living organisms. It’s the primary source of energy for our cells, fueling our bodies’ activities from muscle contractions to brain function. But what makes glucose so special, and why do we refer to it as D-glucose?
The Chirality of Glucose (D-Glucose)
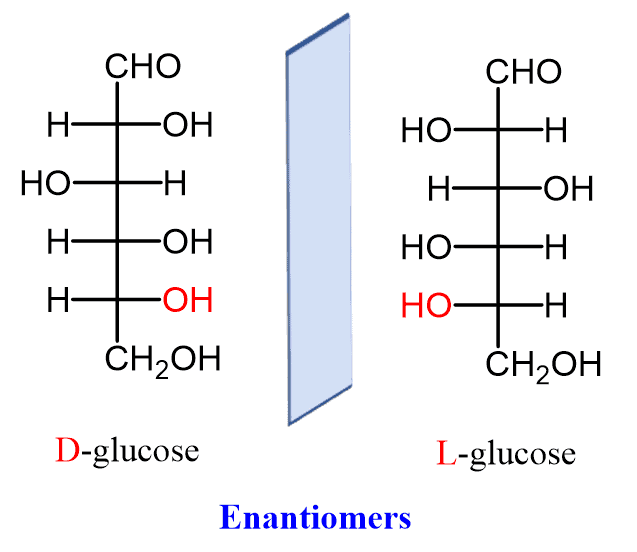
Glucose, like many other sugars, exists in two mirror-image forms: D-glucose and L-glucose. This phenomenon is known as chirality, and it arises from the presence of asymmetric carbon atoms in the molecule. These carbon atoms have four different groups attached to them, leading to two possible spatial arrangements.
D-glucose and L-glucose are enantiomers, meaning they are non-superimposable mirror images of each other. While both forms have the same chemical formula, their three-dimensional structures differ, leading to distinct biological properties.
The D- and L- designations refer to the configuration of the chiral center farthest from the carbonyl group (C=O) in the Fischer projection of the molecule. In D-glucose, this chiral center has the same configuration as D-glyceraldehyde, a simple sugar used as a reference standard.
Why D-Glucose?
Of the two forms, D-glucose is the biologically active form. Enzymes in our bodies are specifically designed to recognize and interact with D-glucose molecules. They are unable to utilize L-glucose, making it essentially useless for our metabolic processes.
The reason for this preference for D-glucose is rooted in the evolutionary history of life on Earth. The first self-replicating molecules likely used D-amino acids and D-sugars. As life evolved, this preference for D-molecules became ingrained in the biochemistry of living organisms.
The Importance of Glucose (D-Glucose)
Glucose plays a crucial role in numerous biological processes:
Energy Production: Glucose is broken down through cellular respiration, generating ATP, the energy currency of cells.
Carbohydrate Metabolism: Glucose is the building block of complex carbohydrates like glycogen and starch, which serve as energy storage molecules.
Biosynthesis: Glucose is used to synthesize other important biomolecules, including amino acids, fatty acids, and nucleotides.
Signaling: Glucose levels in the blood regulate various physiological processes, including hormone secretion and cell growth.
In conclusion, D-glucose is a fundamental molecule of life, providing the energy and building blocks necessary for our survival. Its unique chiral properties and biological significance make it an essential component of our biochemistry.







[…] Read More: D-Glucose: Life’s Sweet Molecule Discussion Tripura Medical College & Hospital […]